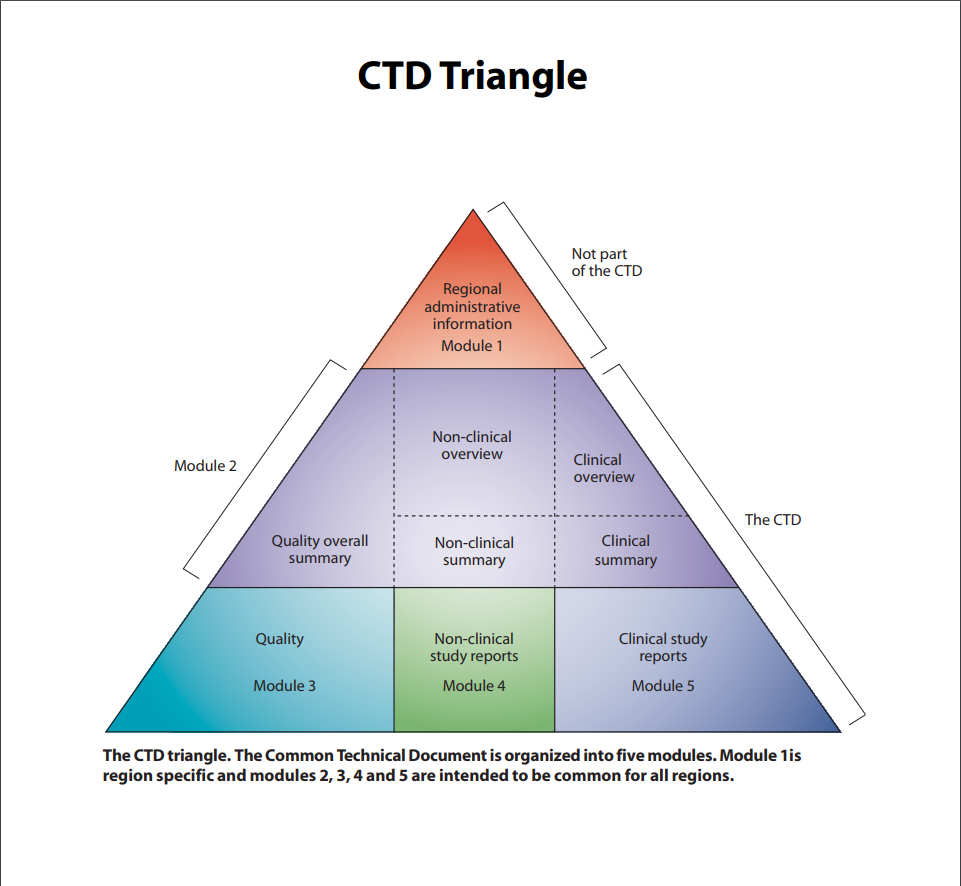
Also called a CTD. The name adopted by the ICH which refers to the way of structuring quality, safety, and efficacy information in support of a marketing authorization application (called a New Drug Application at the FDA). The CTD format is used by the EMA, FDA and Japanese PMDA. The CTD contains five modules (Modules 1, 2, 3, 4, and 5). Clinical Study Reports are contained in Module 5. The CTD is depicted by the ICH as a pyramid.
See http://www.ich.org/products/ctd.html