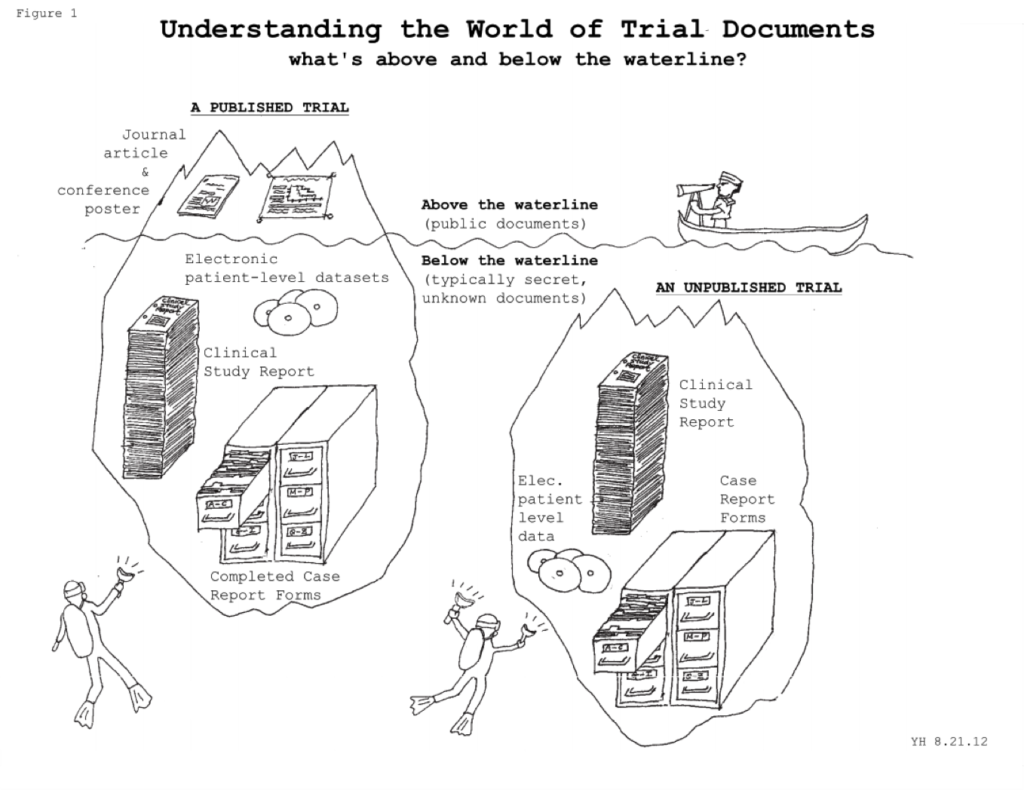
We consider “clinical trial data” to refer to the totality of recorded information related to a clinical trial. Therefore this definition includes not only all datasets from trials, but also the many pre-trial and post-trial documents that are created in the course of designing, running, and reporting a trial (such as the trial protocol, statistical analysis plan, blank and completed case report forms, randomization lists, etc., trial reports e.g. clinical study report, trial publication, and so on). Even meeting minutes, marketing assessments, and email correspondence about trials are included in “clinical trial data,” and may be helpful or even necessary to fully understand a trial.