Health Canada is the federal regulator for drugs and devices in Canada. The regulator has recently shifted its approach to sharing clinical trial information by making Clinical Study Reports (CSRs) available on its website — for free, to anyone, without registration or login. Thanks to Health Canada, CSRs are now a global resource trivially easy to access.
Why care about CSRs?
CSRs are unabridged reports of a clinical study written for regulators. CSRs represent the most complete synthesis of the planning, execution, and results of a clinical trial.
While CSRs share similarities with journal articles (e.g. their structure, including introduction, methods, results, and discussion sections), CSRs are substantially more detailed. They contain numerous tables, figures, and data not constrained by page limits. Individual serious adverse event narratives, for example, are almost never found in publications, but are a standard feature of CSRs. So, too, are pre-study documents like protocols and statistical analysis plans. Other important CSR appendices include blank case report forms (CRFs), certificates of analysis, sample informed consent forms, and individual patient listings. Learn more about CSRs here.
Accessing drug and medical device CSRs from Health Canada
Health Canada is the second regulator (following the European Medicines Agency) that has begun publishing CSRs on its website.
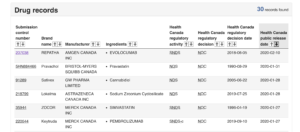
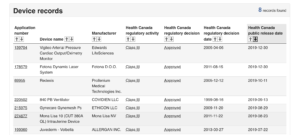
In just two clicks, anybody around the world can freely download trial CSRs from Health Canada’s website. This practice, which began in February 2019, applies to drugs and medical devices and has made Health Canada arguably the world’s most transparent regulator, and the only regulator proactively sharing data on medical devices.
See our website for more details on Health Canada’s new portal, including how you can request data for drugs or medical devices not currently on the portal – as well as information on how to access data from other regulators and non-regulator sources like industry.
CSRs contain important documents for researchers who wish to check on the accuracy and completeness of publications of clinical trials through trial re-analysis. There are also trials that were never published and access to CSRs allows researchers to analyze these trials and publish their findings in order to restore the clinical trial literature.
With the public availability of CSRs, we hope more researchers will be interested in re-analyzing clinical trials so as to contribute to the accuracy of research reporting and the resulting positive impact on the public’s health.

Leave A Comment
You must be logged in to post a comment.