Trial transparency in real time
By: Sarah Tanveer
The focus of clinical trial transparency efforts has been on how to obtain data for completed trials. But when high-profile trials are ongoing, as in the case of Covid-19 drugs and vaccines, there is enormous demand to learn as much as possible about planned and ongoing trials. ClinicalTrials.gov and other trial registers hold some information, but where can one go to learn more? This article discusses some lesser-known and utilized sources where trial details can be found for ongoing, industry sponsored clinical trials.
Investor briefing documents, company press releases, and SEC tax filings are not typical sources medical researchers turn to for learning about trials, but they can provide otherwise unknown details on early stage clinical trials. They also provide updates on pipeline developments of drugs and therapeutics that can be helpful when trying to understand the complexities of ongoing trials, about which very few details are typically available prior to results being published and regulatory approval.
Sponsor and/or Company Website
Many companies have “investor” sections on their website. Here, one can find investor briefing documents, recordings and transcripts of briefings, and press releases. Within these documents, you can often find pieces of key trial information regarding the progress of drugs in the pipeline. Powerpoint presentations, such as this one, update investors on research and development (R&D). They can also give a concise overview of ongoing pivotal trials, information that may not be readily available in traditional sources of information such as clinical trial registries (e.g. Clinicaltrials.gov).
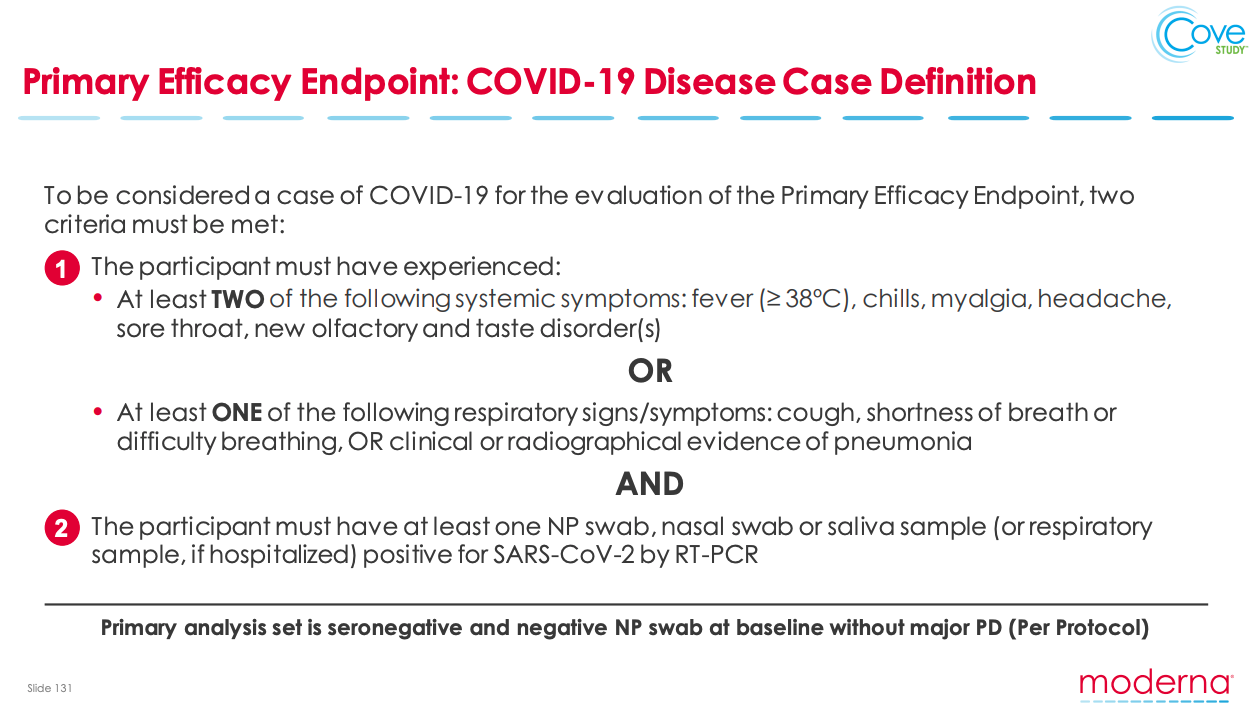
Safety and tolerability data from early stage drug or vaccine development may also be presented within these presentations. Furthermore, primary efficacy endpoints as well as the primary analysis population planned to be used to assess the efficacy of the treatment can sometimes be presented in greater detail than clinical trial registries (Figure 1).
Figure 1: Key trial information provided on a Moderna Investor earnings call that was not provided in the CT.GOV registry entry (August 2020, Slide 131) Source: https://investors.modernatx.com/static-files/8ea64970-8299-43a6-81af-edaf30040fea
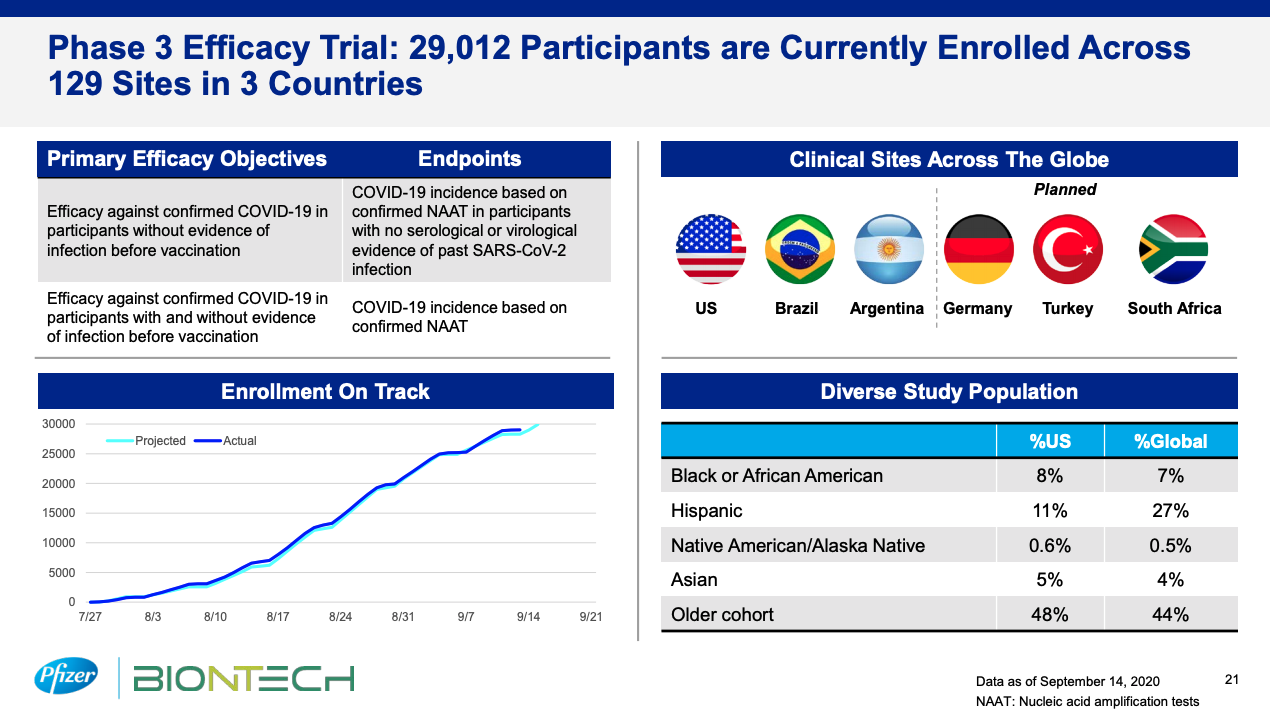
Lastly, updates on trial enrollment and planned interim analysis may also be presented. (Figure 2) Interim analyses can have an impact on the overall trial in the sense that clear positive benefit or clear lack of efficacy may lead to ending the trial early.
Figure 2: Update on recruitment status for Pfizer’s Moderna Investor earning call (September 2020, Slide 21) Source: https://s21.q4cdn.com/317678438/files/doc_presentations/2020/09/Covid-19-Programs_FINAL.pdf
Quarterly (10-Q) and Annual Reports (Form 8K)
In October 2000, the Regulation Fair Disclosure (Regulation FD) was enacted which required that all publicly traded companies make materials shared with investors publicly available.
As a result the US Federal Securities and Exchange Commission (SEC) required that publicly traded companies report information on a regular basis. Quarterly reports can be found on the Form 10-Q and annual reports utilize the Form 8-K on the SEC’s website. Typically the annual report, or Form 8-K, is more comprehensive and in depth than the quarterly reports.
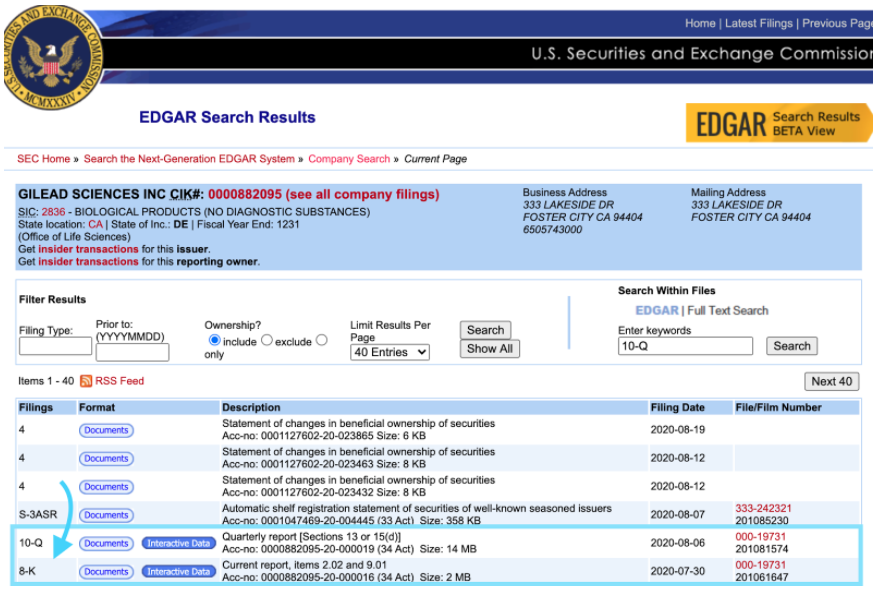
These reports can be found by searching for the company on the SEC EDGAR database and selecting the document of interest. These SEC filings are often also posted on the investor relation section of a company’s website.
Figure 3: Screenshot demonstrating how to access the 10-Q and 8-K document on the US SEC EDGAR database Source: https://www.sec.gov/cgi-bin/browse-edgar?CIK=882095
Other sources to find transcripts and SEC filings
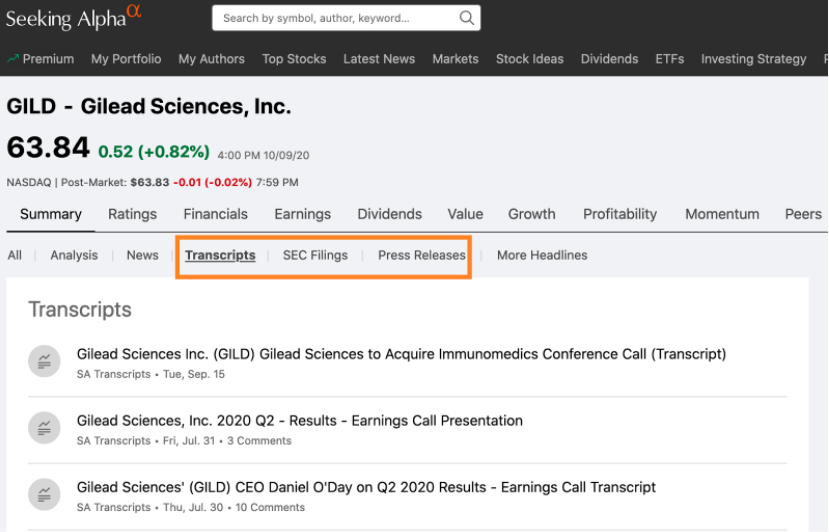
Seekingalpha.com is another resource to quickly locate and find company meeting transcripts, SEC filings, and press releases. You can search for the company of interest, and navigate to the second row of links, and select from the options listed. A free account may be needed to be created to view some documents. If your institution has access to Factiva and Nexis Uni (previously LexisNexis Academic), this information can be found there as well.
Figure 4: SeekingAlpha.com website – results for Gilead Sciences, Inc. Source: https://seekingalpha.com/symbol/GILD
What types of information would be relevant to trialists and researchers in a financial report? These reports can include specific details regarding advancements to clinical drug developments, access, pricing, etc. The speakers on investor earnings calls can also sometimes signal timelines for forthcoming results.
Similar to investor earnings calls, key details regarding a trial’s primary endpoint may be presented. For example, Moderna released details on their primary endpoint for their COVE (mRNA-1273) vaccine trial within their 1O-Q filing in August 2020 (source), more than a month before the company publicly released its trial protocol. At the time, this information was not publicly reported elsewhere. They also reported information regarding planned statistical analysis, including interim analyses, which helped paint a clearer picture of the study design and procedures:
“We are conducting a Phase 3 randomized, 1:1 placebo-controlled study of mRNA-1273, named the COVE study, which began enrollment on July 27, 2020. The study protocol, which has been reviewed by the U.S. Food and Drug Administration (FDA) and is aligned to recent FDA guidance on clinical trial design for COVID-19 vaccine studies, provides for approximately 30,000 participants in the United States at the 100 µg dose level. The primary endpoint will be the prevention of symptomatic COVID-19 disease. Key secondary endpoints include prevention of severe COVID-19 disease (as defined by the need for hospitalization) and prevention of infection by SARS-CoV-2. The primary efficacy analysis will be an event-driven analysis based on the number of participants with symptomatic COVID-19 disease. The target vaccine efficacy (VE) against COVID-19 for powering assumptions is 60% (95% confidence interval to exclude a lower bound >30%). Data will be reviewed by an independent Data Safety Monitoring Board organized by NIH. The trial is expected to have two interim analyses (at approximately 53 and 106 events), prior to a final event-driven analysis at approximately 151 events”


Leave A Comment
You must be logged in to post a comment.